
Oxide surfacesA rusty metal surface is not the most desirable thing in daily life. Certainly everyone would know some maiden aunt spending her days on polishing her silverware. However, oxide surfaces (or surface oxides for that matter) can provide far more interesting problems than simple corrosion protection. Here the keywords are surface chemistry and catalysis. A catalytic surface increases the efficiency of a reaction. The simple-minded view would be that it is much easier for two molecules to find each other when they are attached to a surface rather than flying around in vacuum. This is not quite correct, as the reaction can take place easier in two steps if one of the two components is already present on the surface. This gives us a pointer as to why surfaces covered with oxygen are relevant in catalyzing oxidation reactions. A more complicated phenomenon is related to certain transition metal oxides, which are known to be catalytically active in the presence of metal nanoparticles on the surface. The effect of particle size and dimensionality is even more impressive considering the huge catalytic activity of oxide supported small clusters of Au, which is particularly inert in its bulk form. The details of the reactions taking place on the oxide surface, and the influence of the nanoparticle dimensions are far from being understood in depth. The related phenomena provide a rich playground to combine ideas from surface science and chemistry.
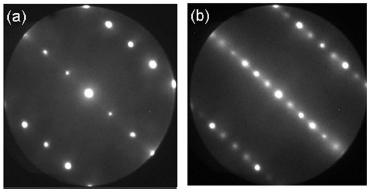 Figure 1: The LEED pattern (a) from a clean (1x1), and (b) from an oxygen deficient (1x2) reconstructed TiO2(110) surface.
Another structural study targeted a particular oxide phase on Ag(111). A full LEED I(V) analysis showed clearly the nature of this phase [4]. (By the way, oxygen adsorption on the silver surface, beyond being an annoyance to the zitella, is relevant for the ethylene epoxidation reaction).
[1] A. Locatelli, T. Pabisiak, A. Pavlovska, T. O. Mentes, L. Aballe, A. Kiejna, E. Bauer, "One-dimensional Au on TiO2 ", J. Phys.:Condens. Matter 19 (2007) 082202.
[2] The process is explained by the Knotek-Feibelman model and it features an interatomic Auger emission, that leaves an oxygen atom positively charged. This oxygen is no more bound to the surface and is easily desorbed.
[3] T. O. Mentes, A. Locatelli, L. Aballe, A. Pavlovska, E. Bauer, T. Pabisiak, A. Kiejna, "Surface modification of oxides by electron-stimulated desorption for growth mode control of metal films: Experiment and DFT calculations", Phys. Rev. B 76, 155413 (2007).
[4] R. Reichelt, S. Gunther, J. Wintterlin, W. Moritz, L. Aballe, T. O. Mentes, "Low energy electron diffraction and low energy electron microscopy microspot IV analysis of the (4x4)O structure on Ag(111): Surface oxide or reconstruction?", J. Chem. Phys. 127, 134706 (2007).
|
